THE TRANSATLANTIC CARDIOVASCULAR STUDY GROUP
The TCV Study Group is a research group dedicated to reduce knowledge gaps in cardiovascular medicine and enhance patient care. It represents a scientific hub built to explore innovative solutions and enhance closer collaboration between investigators by protecting, integrating, and advancing their ideas in medicine. It’s culture is all about translating relevant questions into the right research. It’s goal is to produce unbiased highly relevant research and to identify the conditions and the barriers for desired patient outcomes.
To find out more about the TCV Study Group, you can contact us here.
THE BIO-SHIFT PROJECT
Heart failure (HF) is a global health problem and the leading cause of death and re-hospitalization worldwide. Tools that enable accurate monitoring of clinically silent progression of the disease in an individual patient with HF are critically needed. They could not only improve the risk stratification of HF patients, but also lay the foundation for an individualized therapy potentially improving their life-expectancy as well as their quality-of-life.
The Role of Biomarkers and Echocardiography in Prediction of Prognosis of Chronic Heart Failure Patients (Bio-SHiFT) is a prospective, multi-center cohort study in adult men and women diagnosed with chronic HF visiting the outpatient clinic. This study is designed to investigate whether (and how) disease progression in an individual patient with chronic HF can be accurately monitored by serial measurements of disease-related (novel) biomarkers. Clinical data, blood and urine specimens are taken at the day of inclusion and at prespecified follow-up visits, which are performed every 3 months until the end of the scheduled follow-up. The Bio-SHiFT study also compares 2D- with real-time 3D-echocardiography, and Speckle tracking with tissue Doppler imaging (TDI) in the subgroup of HF patients, and relates these 6-monthly repeated echocardiographic measurements to the patients’ prognosis. The Bio-SHiFT study will provide novel insights into the pathophysiologic processes underlying HF, but also help in identifying the vital signals during clinically silent deterioration of HF that precede adverse clinical outcomes. Identification of these signals will help us to develop the tools to accurately monitor clinically silent disease progression in an individual patient with HF and to derive patient-specific (i.e., individualized) treatment decisions.
To find out more about the Bio-SHiFT project, you can click here.
THE ATHEROREMO-IVUS PROJECT
Acute coronary syndrome (ACS) remains the leading cause of death worldwide. Post-mortem studies have shown that ACS is mostly caused by specific coronary lesions that are at high risk for rupture. Detection of these lesions may be highly relevant for further improvement of prediction, and for optimal choice of treatment in these patients. Yet, the use of standard coronary angiography is limited in detection of these high-risk coronary lesions.
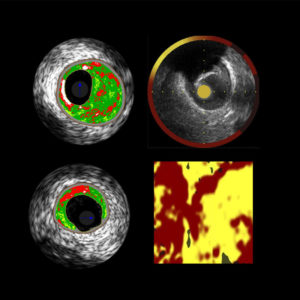
The European Collaborative Project on Inflammation and Vascular Wall Remodeling in Atherosclerosis – Intravascular Ultrasound Study (AtheroRemo-IVUS) aims to investigate the relation of coronary plaque phenotypes and plaque vulnerability as determined by intravascular ultrasound (IVUS) and near infrared spectroscopy (NIRS) with genetic profile and novel circulating biomarkers. The AtheroRemo-IVUS is a prospective, observational, cohort study of patients who underwent coronary catheterization for ACS or stable angina pectoris. Prior to the catheterization procedure, blood samples were drawn for biomarker measurements and genetic analyses. Subsequent to the catheterization procedure, IVUS is performed in a predefined non-culprit coronary artery. In the subgroup of patients, NIRS is additionally performed in the same non-culprit vessel. Primary endpoint is the presence of vulnerable plaque as determined by IVUS-virtual histology. Secondary endpoint is long-term incidence of major adverse cardiac events. AtheroRemo-IVUS study will improve our knowledge on the role of genetic profile and inflammation in the development of atherosclerosis and vulnerable plaques. Likewise, novel biomarkers and intracoronary imaging techniques will be validated in this study.
To find out more about the AtheroRemo-IVUS project, you can click here.
THE BIOMARCS PROJECT
Progression of coronary artery disease (CAD) towards acute coronary syndrome (ACS) is believed to be a dynamic process with multiple constituents in which a plaque destabilizing sequences lead to repeated ACS within a specific time-frame. However, current single-assessment risk models are inadequate to identify these periods of increased coronary vulnerability within a precise, short time-frame at an individual patient level. Yet, knowing these temporal biomarkers’ patterns prior and following ACS could provide novel insights into the pathophysiologic processes underlying progression of CAD towards ACS.
The BIOMarker study to identify the Acute risk of a Coronary Syndrome (BIOMArCS) was designed to evaluate whether serial measurements of multiple (novel) biomarkers can predict such “vulnerable periods”. The BIOMArCS is a multicentre, prospective, observational study conducted in 18 participating hospitals in the Netherlands. Blood samples were collected at admission, hospital discharge, and every two weeks after index ACS during the first six months, followed by monthly collection until one year. To enable a precise description of early washout biomarker patterns, a subgroup of BIOMArCS patients underwent additional blood sampling at 24, 48, 72 and 96 hours after the index (first) ACS. The BIOMArCS study aims to identify appropriate biomarkers that are associated with the constituents and the development of ACS, but also to study and describe the variability and evolution patterns of different circulating biomarkers. A subsequent aim of this study is to create an infrastructure for repeated biomarker sampling that can easily be expanded to perform future projects involving biomarkers in CAD, in order to eventually evaluate the use of biomarkers as a non-invasive, cost effective and accessible method of individual risk stratification in the dynamic processes of vascular inflammation, endothelial activation, atherogenesis, as well as the onset of ACS.
To find out more about the BIOMArCS project, you can click here.
CONGENITAL VASCULAR MALFORMATIONS
Congenital vascular malformations are structural irregularities of the vasculature which occur due to developmental defects during various stages of embryogenesis. Unlike vascular tumors (e.g. hemangioma), congenital vascular malformations are present at birth and grow proportionally with the individual growth without spontaneous involution. A vascular malformation is first classified by a predominant vascular defect (arterial, venous, arteriovenous, lymphatic, capillary, or combined) and subsequently divided into truncular or extratruncular form depending on the developmental arrest in the earlier stages of embryonal life, i.e., ‘‘undifferentiated capillary network to reticular/plexifrom network stages’’ (extratruncular) or at the later stages of embryonal life, i.e., ‘‘maturation period’’ (truncular).
Parkes Weber syndrome represents complex combined congenital vascular malformations which consists of capillary malformation (CM), venous malformation (VM), lymphatic malformation (LM), and arteriovenous malformation (AVM) in the overgrowth limb. To find out more on diagnostic and treatment modalities of this syndrome you can click here.